On my return from the Ruelle gun-foundry I resumed my experiments with the open-hearth furnace, when the remarkable incident, mentioned above, occurred in this way. Some pieces of pig iron on one side of the bath attracted my attention by remaining unmelted in the great heat of the furnace, and I turned on a little more air through the fire-bridge with the intention of increasing the combustion. On again opening the furnace door, after an interval of half an hour, these two pieces of pig still remained unfused. I then took an iron bar, with the intention of pushing them into the bath, when I discovered that they were merely thin shells of decarburised iron, as represented at A, Fig. 37, Plate XII, showing that atmospheric air alone was capable of wholly decarburising grey pig iron, and converting it into malleable iron without puddling or any other manipulation. Thus a new direction was given to my thoughts, and after due deliberation I became convinced that if air could be brought into contact with a sufficiently extensive surface of molten crude iron, it would rapidly convert it into malleable iron. This, like all new problems, had a special interest for me, and I became impatient to test it by a laboratory experiment. Without loss of time I had some fire-clay crucibles made with dome-shaped perforated covers, and also with some fire-clay blowpipes, which I joined on to a 3 ft. length of 1-in. gas-pipe, the opposite end of which was attached by a piece of rubber tubing to a fixed blast-pipe. This elastic connection permitted of the blow-pipe being easily introduced into and withdrawn from the crucible, as shown at Fig. 38, Plate XIII., which represents a vertical section of an air furnace containing a crucible that, in this case, forms the "converter."
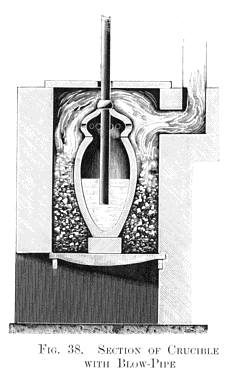
About 10 lb. of molten grey pig iron half filled the crucible, and thirty minutes' blowing was found to convert 10 lb. of grey pig into soft malleable iron. Here at least one great fact was demonstrated, viz., the absolute decarburisation of molten crude iron without any manipulation, but not without fuel, for had not a very high temperature been kept up in the air furnace all the the time this quiet blowing for thirty minutes was going on, it would have resulted in the solidification of the metal in the crucible long before complete decarburisation had been effected. Hence arose the all-important question: can sufficient internal heat be produced by the introduction of atmospheric air to retain the fluidity of the metal until it is wholly decarburised in a vessel not externally heated? This I determined to try without delay, and I fitted up a larger blast- cylinder in connection with a 20 horse-power engine which I had daily at work. I also erected an ordinary founder's cupola, capable of melting half a ton of pig iron. Then came the question of the best form and size for the experimental "converter." I had very little data to guide me in this, as the crucible converter was hidden from view in the furnace during the blow. I found, however, that slag was produced during the process, and escaped through the holes to the lid. Owing to this, I determined on constructing a very simple form of cylindrical converter, about 4 ft. in height in the interior, which was sufficiently tall and capacious, as I believed, to prevent anything but a few sparks and heated gases from escaping through a central hole made in the flat top of the vessel for that purpose, as shown in the vertical section at Fig. 39, Plate XIII.
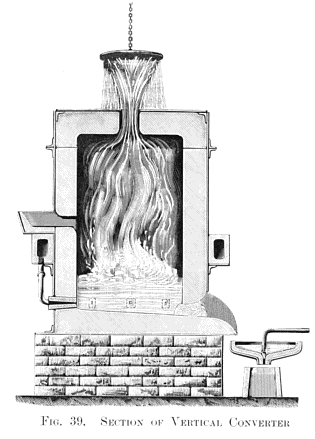
The converter had six horizontal tuyéres arranged around the lower part of it; these were connected by six adjustable branch pipes, deriving their supply of air from an annular rectangular chamber, extending around the converter, as shown.
All being thus arranged, and a blast of 10 or 15 lb. pressure turned on, about 7 cwt. of molten pig iron was run into the hopper provided on one side of the converter for that purpose. All went on quietly for about ten minutes; sparks such as are commonly seen when tapping a cupola, accompanied by hot gases, ascended through the opening on the top of the converter, just as I supposed would be the case. But soon after a rapid change took place; in fact, the silicon had been quietly consumed, and the oxygen, next uniting with the carbon, sent up an ever-increasing stream of sparks and a voluminous white flame. Then followed a succession of mild explosions, throwing molten slags and splashes of metal high up into the air, the apparatus becoming a veritable volcano in a state of active eruption. No one could approach the converter to turn off the blast, and some low, flat, zinc-covered roofs, close at hand were in danger of being set on fire by the shower of red-hot matter falling on them. All this was a revelation to me, as I had in no way anticipated such violent results. However, in ten minutes more the eruption had ceased, the flame died down, and the process was complete. On tapping the converter into a shallow pan or ladle, and forming the metal into an ingot, it was found to be wholly decarburised malleable iron.
| Previous chapter/page | Back | Home | Email this | Search | Discuss | Bookmark | Next chapter/page |