-
to the sun, and the electrons to the planets. Each kind of atom -- each chemical element has a different quorum of planet electrons. Our own solar system with eight planets might be compared especially with the atom of oxygen which has eight circulating electrons. In terrestrial physics we usually regard the girdle or crinoline of electrons as an essential part of the atom because we rarely meet with atoms incompletely dressed; when we do meet with an atom which has lost one or two electrons from its system, we call it an 'ion'. But in the interior of a star, owing to the great commotion going on, it would be absurd to exact such a meticulous standard of attire. All our atoms have lost a considerable proportion of their planet electrons and are therefore ions according to the strict nomenclature.
Ionization of Atoms
At the high temperature inside a star the battering of the particles by one another, and more especially the collision of the ether waves (X-rays) with atoms, cause electrons to be broken off and set free. These free electrons form the third population to which I have referred. For each individual the freedom is only temporary, because it will presently be captured by some other mutilated atom; but meanwhile another electron will have been broken off somewhere else to take its place in the free population. This breaking away of electrons from atoms is called ionization, and as it is extremely important in the study of the stars I will presently show you photographs of the process.My subject is 'Stars and Atoms'; I have already shown you photographs of a star, so I ought to show you a photograph of an atom. Nowadays that is quite easy. Since there are some trillions of atoms present in the tiniest piece of material it would be very confusing if the photograph showed them all. Happily the photograph exercises discrimination and shows only 'express train' atoms which flash past like meteors, ignoring all the others. We can arrange a particle of radium to shoot only a few express atoms across the field of the camera, and so have a clear picture of each of them.
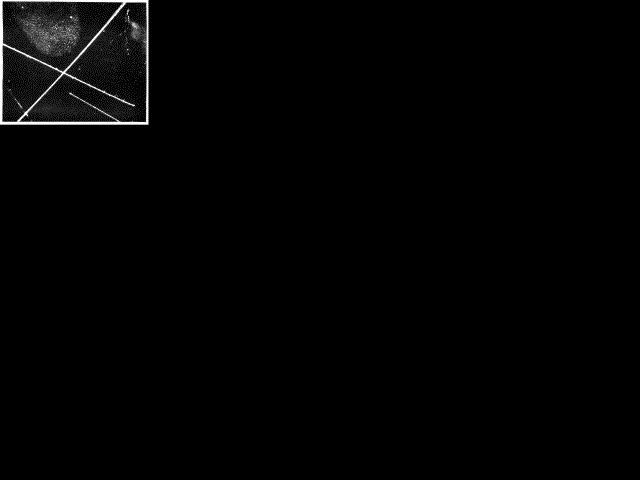 Fig 3. Tracks of Alpha Particles (helium atoms). (C. T. R. Wilson)
Fig 3. Tracks of Alpha Particles (helium atoms). (C. T. R. Wilson) Fig. 3 is a photograph of three or four atoms which have flashed across the field of view-giving the broad straight tracks. These are atoms of helium discharged at high speed from a radio-active substance.
I wonder if there is an under-current of suspicion in your minds that there must be something of a fake about this photograph. Are these really the single atoms that are showing themselves -- those infinitesimal units which not many years ago seemed to be theoretical concepts far outside any practical apprehension? I will answer that question by asking you one. You see a dirty mark on the picture. Is that somebody's thumb ? If you say Yes, then I assure you unhesitatingly that these streaks are single atoms. But if you are hypercritical and say 'No. That is not anybody's thumb, but it is a mark that shows that somebody's thumb has been there', then I must be equally cautious and say that the streak is a mark that shows where an atom has been. The photograph instead of being the impression of an atom is the impression of the impression of an atom, just as it is not the impression of a thumb but the impression of the impression of a thumb. I don't see that it really matters that the impression is second-hand instead of first-hand. I do not think we have been guilty of any more faking than the criminologist who scatters powder over a finger-print to make it visible, or a biologist who stains his preparations with the same object. The atom in its passage leaves what we might call a 'scent' along its trail; and we owe to Professor C. T. R. Wilson a most ingenious device for making the scent visible. Professor Wilson's 'pack of hounds' consists of water vapour which flocks to the trail and there condenses into tiny drops.
 Fig 4. Tracks of Beta Particles (electrons). (C. T. R. Wilson)
Fig 4. Tracks of Beta Particles (electrons). (C. T. R. Wilson) You will next want to see a photograph of an electron. That also can be managed. The broken wavy trail in Fig. 3 is an electron. Owing to its small mass the electron is more easily turned aside in its course than the heavy atom which rushes bull-headed through all obstacles. Fig. 4 shows numerous electrons, and it includes one of very high speed which on that account was able to make a straight track. Incidentally it gives away the device used for making the tracks visible, because you can see the tiny drops of water separately.
 Fig 5. Ionization by X-rays. (C. T. R. Wilson).
Fig 5. Ionization by X-rays. (C. T. R. Wilson).
| Previous chapter/page | Back | Home | Email this | Search | Discuss | Bookmark | Next chapter/page |